Understanding longevity: From gene sequences to social inequity
03 April 2025
Published online 25 March 2022
A causal link between obesity, liver inflammation and glucose disruption could provide a new therapeutic target.
Clues to the root cause of dysglycemia in obesity have now been uncovered by an international team of researchers, including scientists at the Dasman Diabetes Institute in Kuwait.
“Most previous studies suggest that proinflammatory cytokines secreted by infiltrating immune cells in the liver are primarily to blame for the altered metabolic state in patients,” says Suraj Patel of University of Texas Southwestern Medical Center in the US. “However, anti-inflammatory treatments targeting these cytokines have consistently proven ineffective at improving insulin action and metabolic function in humans.”
The team set out to determine how inflammation causes metabolic dysfunction, hoping to identify new targets for the treatment of obesity and diabetes.
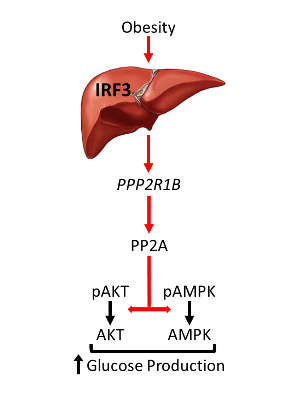
Using mouse models, they found that an innate immune response molecule called interferon regulatory factor 3 (IRF3), which is highly active in the liver during high-fat diet consumption, directly regulates glucose homeostasis. IRF3 activates a gene called Ppp2r1b, which increases the activity of an enzyme complex, leading to glucose over-production and associated insulin resistance.
“Suppressing this axis in our mouse models reversed insulin resistance and restored glucose homeostasis in the liver,” says Patel. Direct targeting of IRF3 in liver cells protected against dysglycemia specifically, whereas targeting IRF3 across the whole body provided protection against dysglycemia and fat accumulation. “Our work suggests a novel therapeutic approach for limiting the high blood sugars that accompany obesity,” says Evan Rosen at Harvard Medical School in Boston, US.
“We are indebted to our long-standing collaboration with the group at Dasman Diabetes Institute,” adds Rosen. “They produced beautiful histological images that we used to evaluate immune cell infiltration into the liver due to obesity. These images helped us demonstrate that IRF3-deficient mice are protected from hepatic immune cell recruitment during high-fat diet consumption.”
doi:10.1038/nmiddleeast.2022.14
Patel, S.J. et al. Hepatic IRF3 fuels dysglycemia in obesity through direct regulation of Ppp2r1b. Sci. Transl. Med. 14, eabh3831 (2022).
Stay connected: